High temperatures in Texas have been above 100 for over a month. When we park our car to go shopping, there is rarely any tree shade in which to place the car. As a result, the car is left in the direct glare of the sun, with windows closed (since we don’t like the idea of car theft). We go shopping. When we step from the mall to the parking lot, we experience a temperature difference of about 35 degrees Fahrenheit (going from an air-conditioned building at around 70 degrees to a local atmospheric temperature of 105). When we open the door of the car and jump inside, our bodies experience a FURTHER 25-degree temperature difference, this one between the ambient outdoor temperature (105) and the inside of the car (about 130 degrees). But wait . . . hasn’t the car been sitting in the air, coming into thermal equilibrium with the surroundings? Yes, but the total heat energy in the car is not simply that due to the energy entering the vehicle from the sun or from thermal contact with the atmosphere; it’s much higher, due to the heat-trapping action of the greenhouse effect.
Let us explore how the greenhouse effect works in a car. Solar radiation passes through the windows in the car and is absorbed by the materials in the car – the seats, the dashboard, the carpets, etc. Light is made from photons. For each photon, there is a direct relationship between energy and wavelength:
E = h/λ
Here, h is Planck’s Constant: h = 6.626 x 10-34 J ⋅ s. If, in a beam of solar radiation, you have more photons present with short (small) wavelengths, then the beam of light carries more overall energy than if it is composed primarily of long (large) wavelength photons. What is the composition of wavelengths of light before it enters the glass?
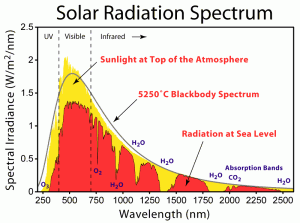
Light which has reached the surface of the earth has a composition as shown left [1]. Focus on the red-colored distribution; we see that a great fraction of the spectrum is concentrated in the visible portion of the spectrum, between 450-700 nm. Most of the spectrum lies in the infrared – the portion of the spectrum whose energy we feel as heat. These are wavelengths above 700nm. The remainder of the spectrum is ultraviolet – short wavelengths, below 450nm.
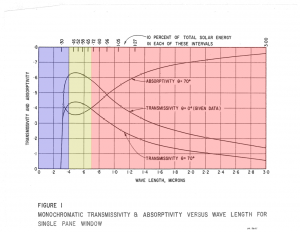
This is the spectrum of solar radiation that reaches our windshield and our passenger and rearview windows. These transparent materials are really transparent only to what we consider “visible” light, as illustrated by the graphic on the left [2].
Since most of the solar radiation enters as heat, and glass strongly absorbs heat, this leads to the glass re-radiating heat energy in all directions; some of it enters the car, the rest returns to the environment. Visible solar radiation is readily transmitted through the glass; this is absorbed by the materials in the car, while some of it (the colors we see) are reflected as visible light. The energy absorbed by materials in the car is re-radiated as heat energy (infrared). Some of this reaches the glass and is again primarily absorbed rather than transmitted. The glass then re-radiates the heat energy in all directions, some re-entering the car and some leaving the car.
We see how the equilibrium situation reached by the inside of the car is not represented by the external temperature; most of the sun’s radiation enters the car as heat, and most of that is trapped by the glass. The visible light that enters the car is primarily re-radiated as heat, and most of that is also trapped by the glass. So there is more heat energy trapped in the car than would be without the physical and chemical tendency of glass to absorb and trap heat energy. This is why a hot car on a hot day is at a higher internal temperature than the ambient air, and this is the essence of the greenhouse effect.
So where does this leave us with our great automobile, the planet Earth, on whose skin we ride in an endless waltz around our central star? There is no glass that surrounds Earth, but there is an atmosphere. What are the radiation transmission and absorption coefficients of our atmosphere?
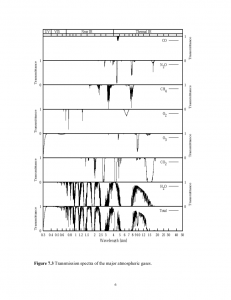
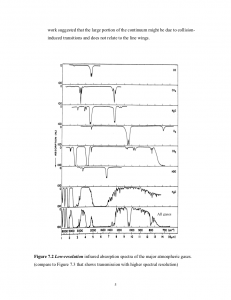
The graphs above show the transmittance (top) and absorption (bottom) of radiation as a function of wavelength [3].Transmittance is shown for many common atmospheric gases all the way from UV to thermal infrared. Absorption is shown for these same gases, but only for the thermal infrared portion of the spectrum (heat energy). Keep in mind that the sum of transmittance and absorption must equal 100% (1.0); that is, when light strikes a material it must either be absorbed or transmitted – there are no other options. So if a gas has a high transmittance, it necessarily will have a low absorption.
Let’s choose a single gas and discuss its measured properties. Let’s choose water (H20). Water transmits nearly all visible wavelengths of light. It transmits parts of the infrared spectrum, but absorbs wide portions of that spectrum; it therefore is a good “greenhouse gas” – one which readily absorbs infrared (heat). Looking closely at the absorption curves in the infrared, we see this property reinforced (the absorption is highest when the curve is near the bottom of each graph).
How about CO2? We see that CO2 transmits a lot more of the infrared portion of the spectrum, but it has segments where its absorption is very strong. Looking closely at the absorption, we see this confirmed. CO2 is less strong of a greenhouse gas compared to water, but it’s a good contender. But, except for methane (CH4), it’s a vastly superior greenhouse gas compared to other things in the atmosphere: CO, O2, O3, etc.
We need no glass to cause absorption of the heat energy re-radiated by the surface of the earth; we have greenhouse gases, which fill the same purpose as the glass in our car windows. What is the gas composition of the earth’s atmosphere? It is as follows [4]:
- N2: 78.084%
- O2: 20.946%
- Ar: 0.9340%
- H20: ~0.4% (varies with height in the atmosphere – closer to the surface it varies between 1-4%)
- CO2: 0.039%
and other gases. Of the greenhouse gases, CO2 is second in concentration only to water. Water vapor clearly plays an overall larger role in the fact that our surface is warmer than expected due to incident solar radiation.
Let’s consider one last question before we close this post. If H20 is such a stupendous greenhouse gas, surely a little CO2 can’t be causing all this warming?
We’ll explore that question in the next post.
[1] http://en.wikipedia.org/wiki/Sunlight#Composition
[2] Mitalas, G. P. and Stephenson, D. G. “Absorption and Transmission of Thermal Radiation by Single and Double Glazed Windows.” 1962. http://www.nrc-cnrc.gc.ca/obj/irc/doc/pubs/rp/rp173/rp173.pdf
[3] http://www.heliosat3.de/e-learning/radiative-transfer/rt/Lecture-7.pdf

